
At 300 K, 36 g of glucose present per litre in its solution has an osm
pi=CRT" (C = molar concentration)" (pi(1))/(pi(2))=(C(1))/(C(2))," "(4.98)/(1.52)=(36//180)/(C(2))" or "C(2)=(36)/(180)xx(1.52)/(4.98)="0.061 M"

2.22At300 K,36 g of glucose present in a litre of its solution has an osm..

Based on solute - solvent interactions, arrange the following in order

If the elevation in boiling point of a solution of 10 g of solute (mol
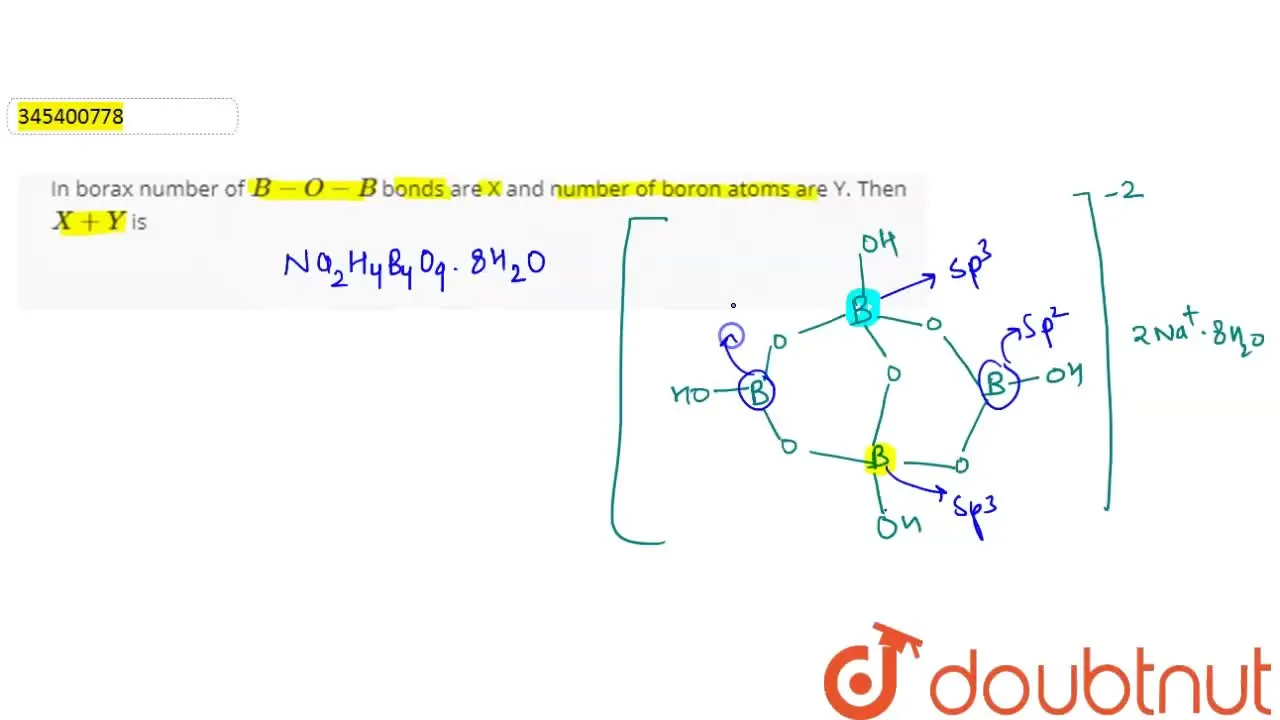
In borax number of B-O-B bonds are X and number of boron atoms are Y.
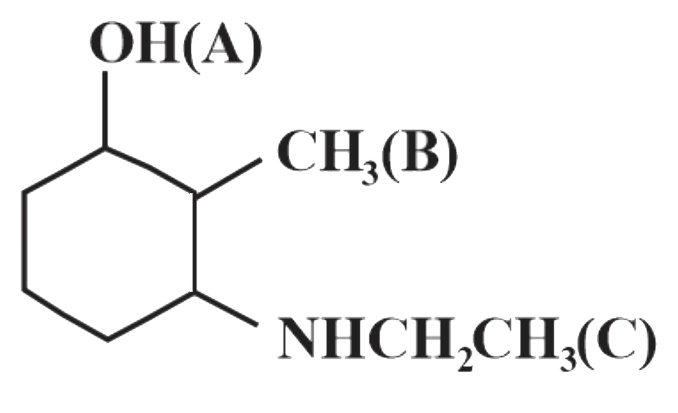
The current carrying ions are not necessarily discharged at the electr

At 300 K, 36 gof glucose present in a litre of its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bars the same

At 300 K 36 g of glucose present in a litre of its solution has an osmotic pressure of 4.98 bar.
At 300 K, 36 g of glucose present per litre in its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of solution - Sarthaks eConnect

Calculations Pharma, PDF, Solution

What role does the molecular interaction play in a solution of alcohol
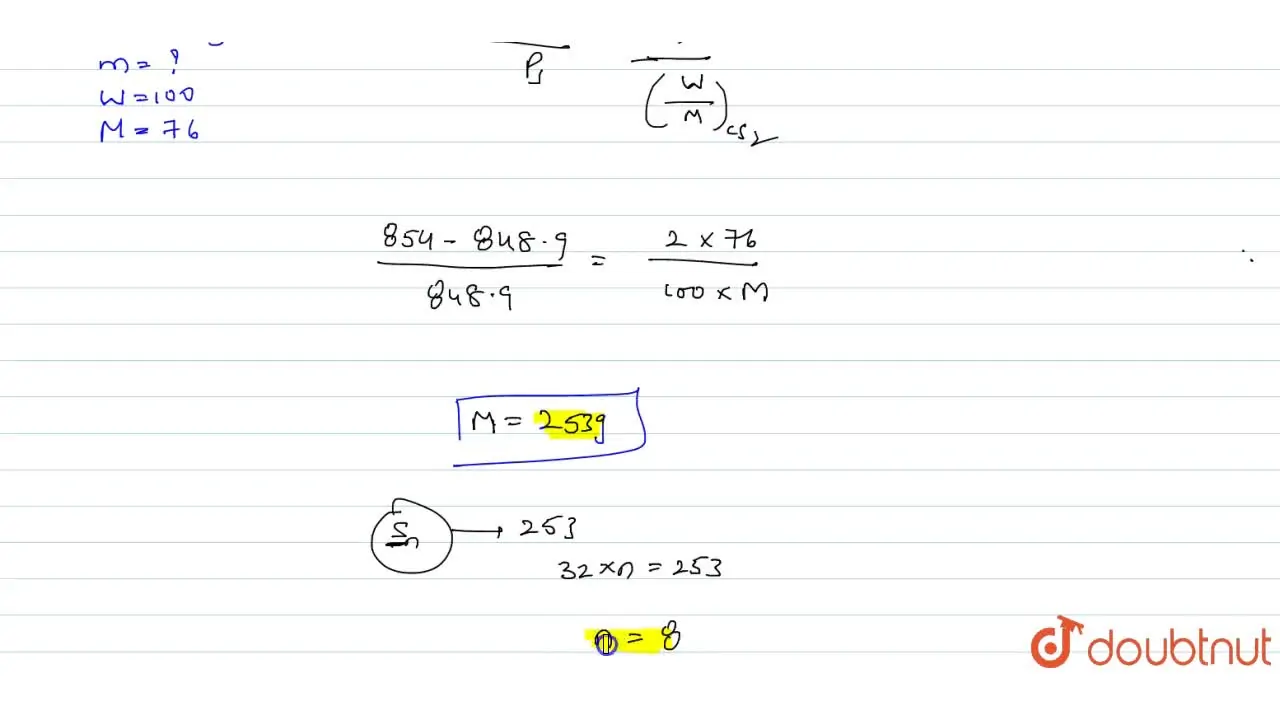
The vapour pressure of CS(2) at 50^(@)C is 854 torr and a solution o

Find the molaity of water. Given: rho =1000kg//m^(3) [Report your
)








