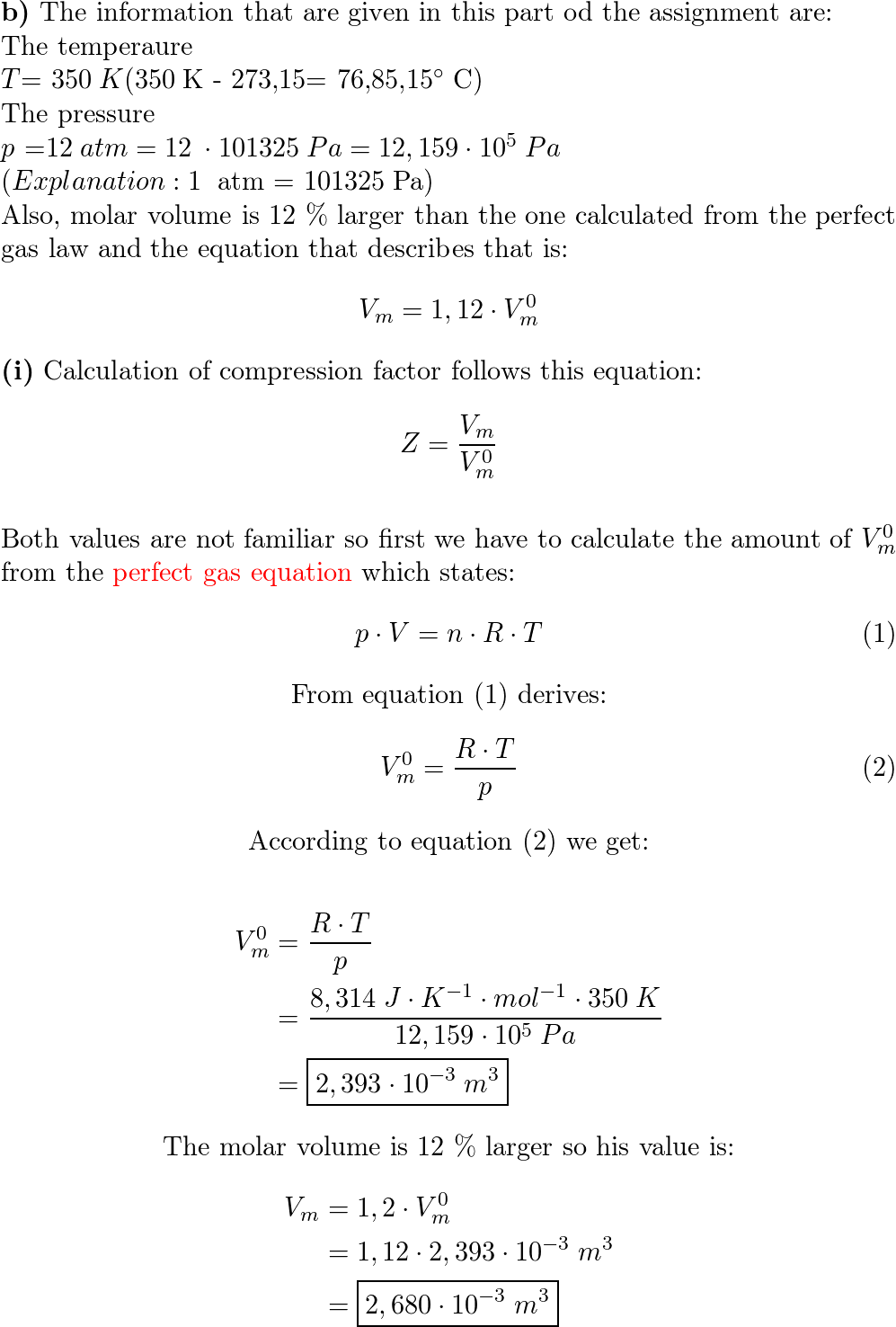
The compressiblity factor a gas obeying van der Waals' equation of state is given by V V-b RTV (2) a ✓ RTV V-b V-b RTV (3) Va (4) RTV V-6
Click here:point_up_2:to get an answer to your question :writing_hand:the compressiblity factor for a gas obeying vander waals equation of state is given byvvbrtv2
Click here👆to get an answer to your question ✍️ The compressiblity factor a gas obeying van der Waals- equation of state is given by V V-b RTV -2- a - RTV V-b V-b RTV -3- Va -4- RTV V-6
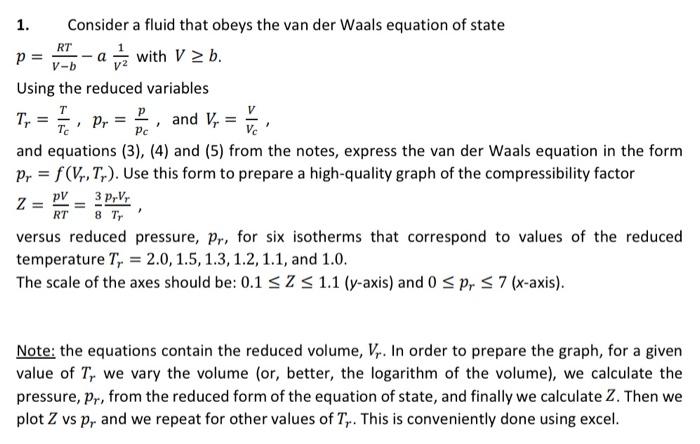
1. Consider a fluid that obeys the van der Waals

Knjiga, PDF, Fluid Dynamics
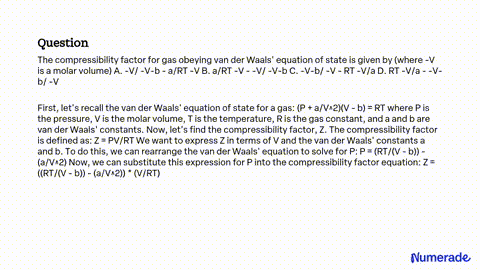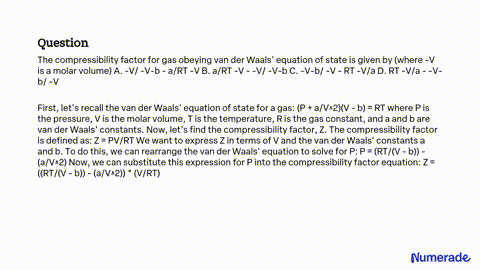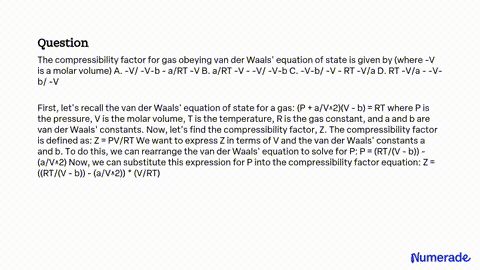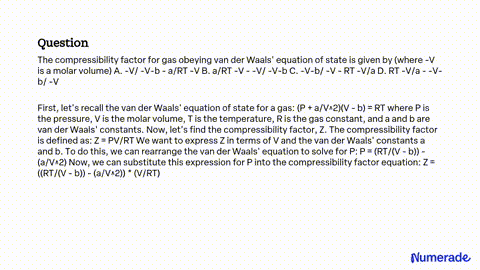
SOLVED: The compressibility factor for gas obeying van der Waals' equation of state is given by (where -V is a molar volume) A. -V/ -V-b - a/RT -V B. a/RT -V -

Compressibility Factors for van der Waals Gases - Wolfram Demonstrations Project

Real gases 1.4 Molecular interactions 1.5 The van de Waals equation 1.6 The principle of corresponding states Real gases do not obey the perfect gas law. - ppt download

Thermodynamics [1 ed.] 0074620142, 9780074620144

Assertion :Compressibility factor Z according to van der Waal's equation may be written as Z=cfrac {1}{1-(cfrac {nb}{V})}-cfrac {an}{RTV}. Reason: For real gases Z > < 1.Both Assertion and Reason are correct and

PDF) Part 1: Equilibrium Erik Roberto Souza
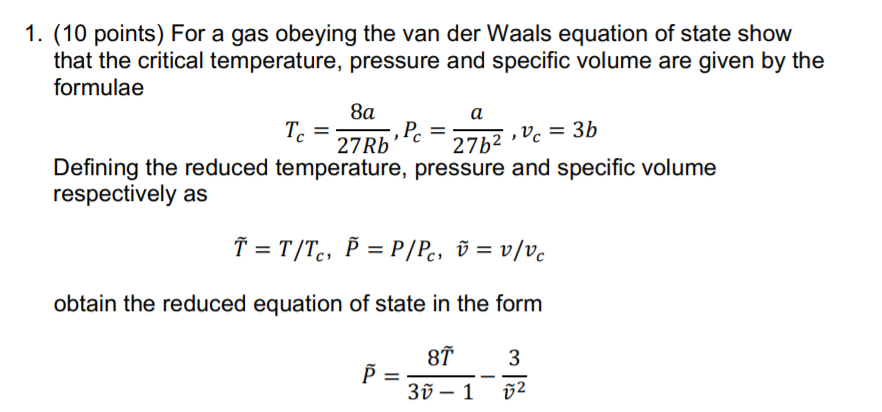
Solved 1. (10 points) For a gas obeying the van der Waals

Compressibility of a van der Waals Gas, Physical Chemistry I
Engineering.Mechanics.STATICS.Fourteenth.Edition