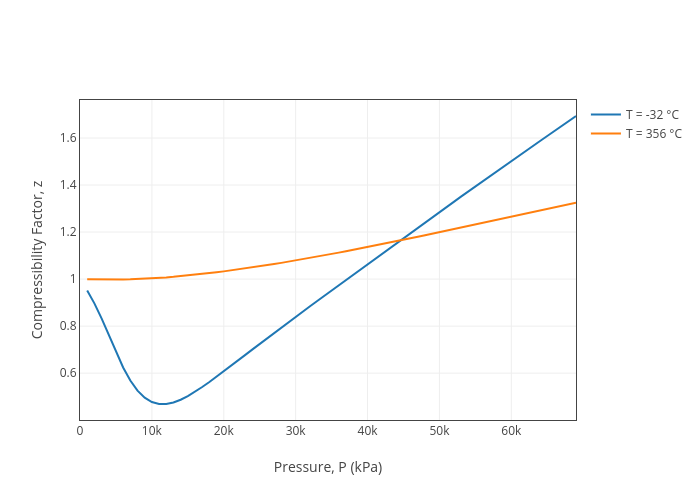
For $CO$, isotherm is of the type as shown. Near the point compressibility factor $Z$ is?\n \n \n \n \n 1.$\\left( {1 + \\dfrac{b}{V}} \\right)$ 2.$\\left( {1 - \\dfrac{b}{V}} \\right)$3.$\\left( {1 + \\
For $CO$, isotherm is of the type as shown. Near the point compressibility factor $Z$ is?\n \n \n \n \n 1.$\\left( {1 + \\dfrac{b}{V}} \\right)$ 2.$\\left( {1 - \\dfrac{b}{V}} \\right)$3.$\\left( {1 + \\dfrac{a}{{RTV}}} \\right)$4.$\\lef

Isentropic compressibility for ideal gas

Compressibility factor - Wikipedia

Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with Compressibility Factor

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

52. For CO, isotherm is of the as shown. Near the point A, compressibility factor Z is CO Ideal

Solved 2. (20 points) At low pressures, the compressibility

Assertion: Compressibility factor `(Z)` for non ideal gases is always greater than `1`.

For $CO$, isotherm is of the type as shown. Near the point compressibility factor $Z$ is? 1.$\left( {1 + \dfrac{b}{V}} \right)$ 2. $\left( {1 - \dfrac{b}{V}} \right)$3.$\left( {1 + \
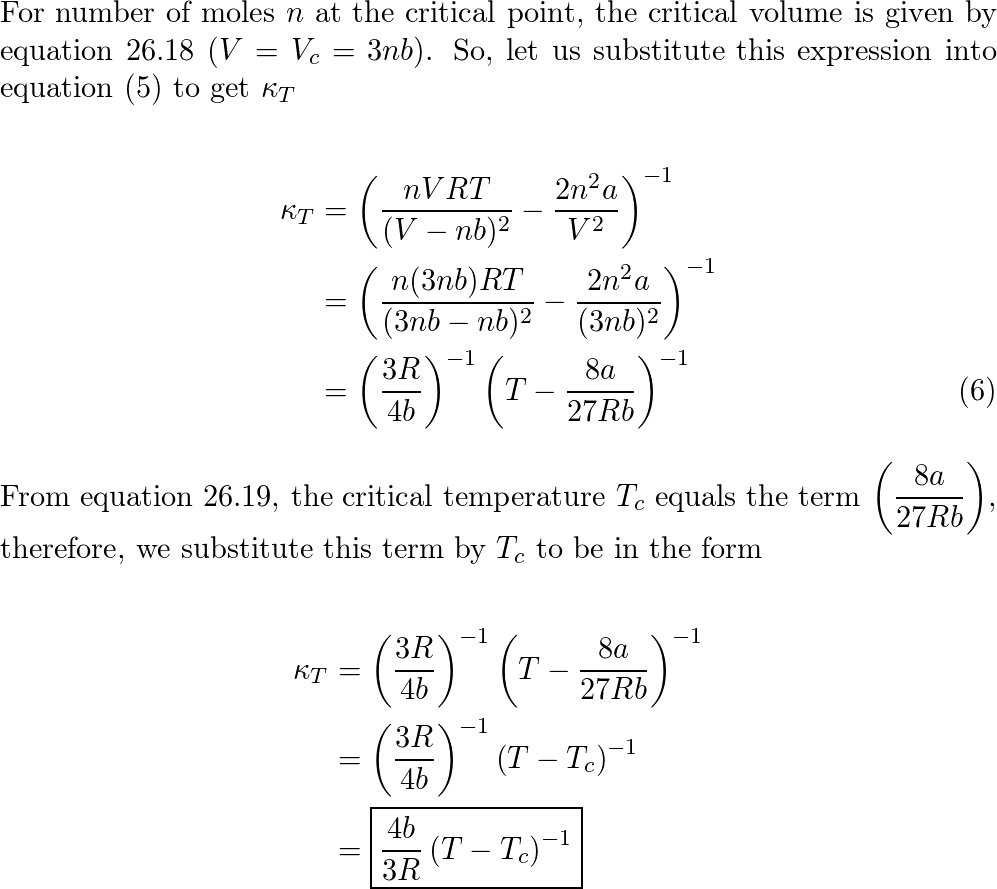
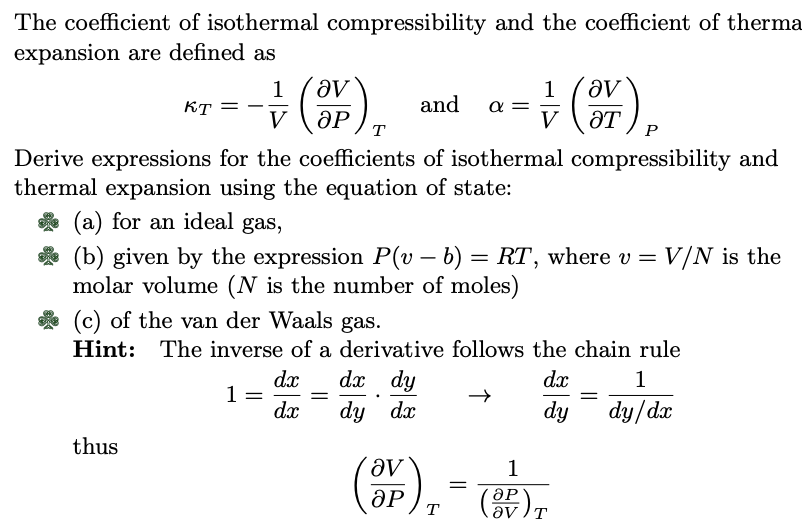
Solved () tor α = (*), P The coefficient of isothermal

Real Gas Behavior The Compression Factor (Z) [Example #2]