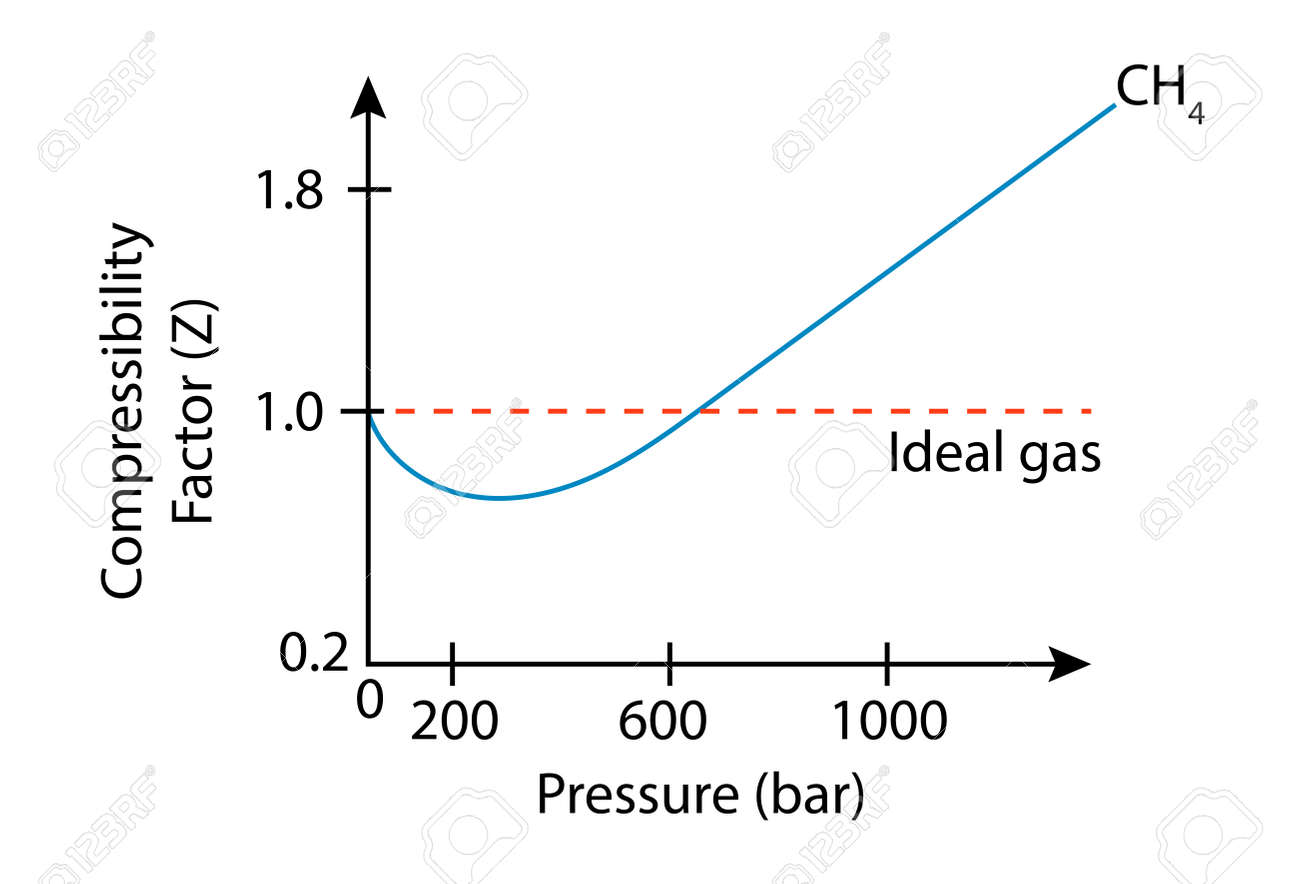
physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In

temperature - In a liquid-in-glass thermometer, how does the gas
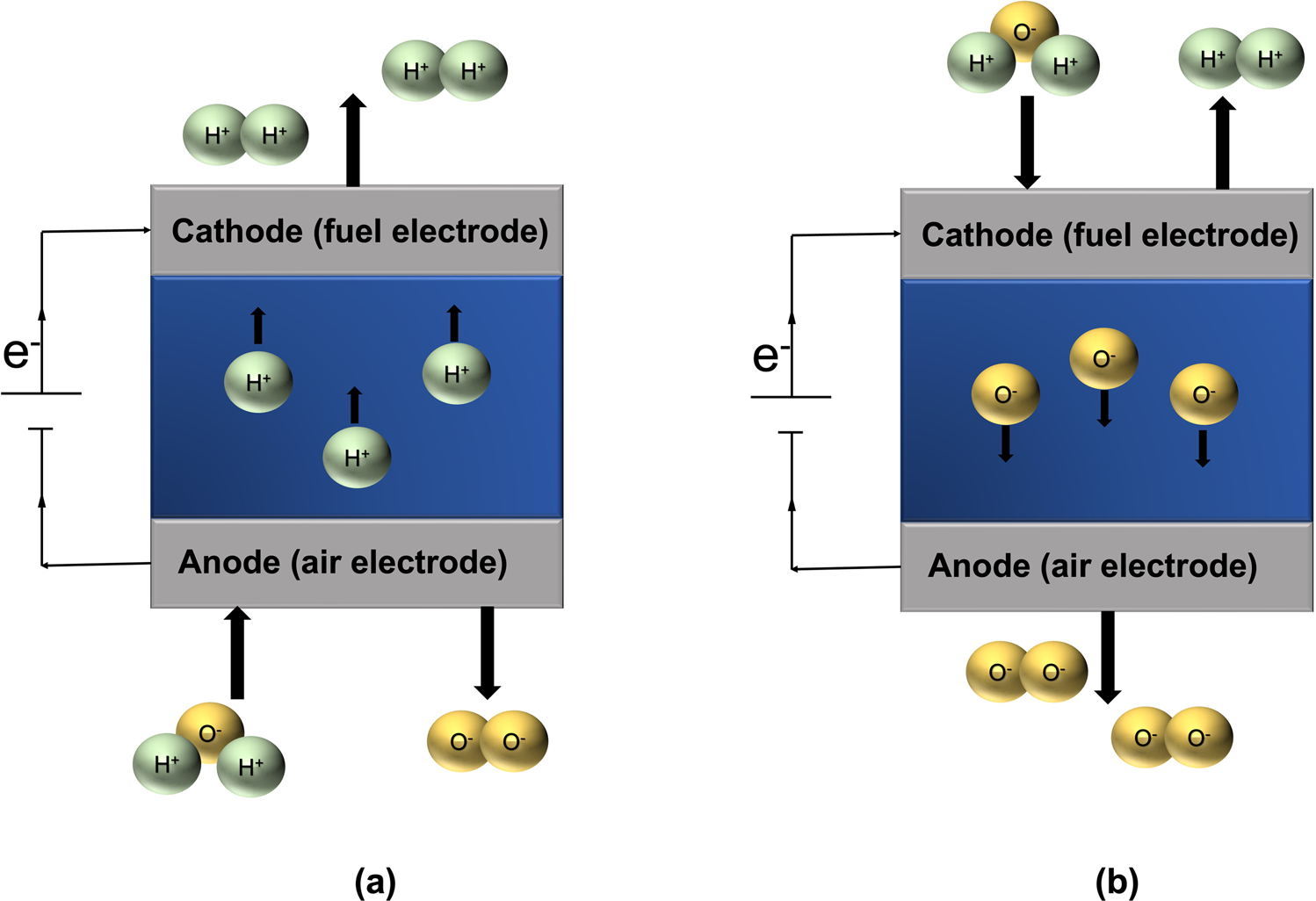
Enhancing the Faradaic efficiency of solid oxide electrolysis

What Exactly is The Compressibility of Fluids?
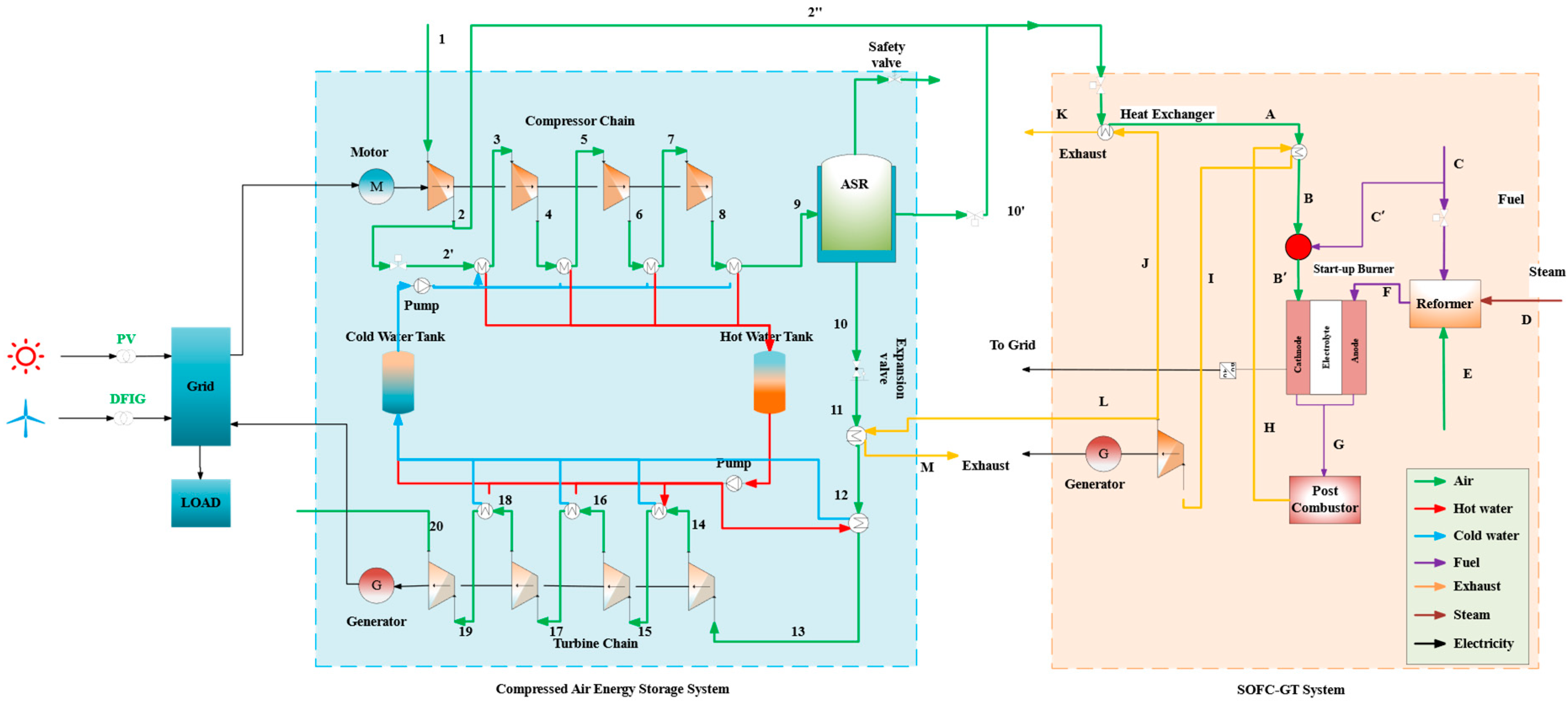
Energies, Free Full-Text

Thermodynamic analysis of a zero-emission combustion cycle for

Why do gases show non ideal behavior at low temperature and high

Heat pump - Wikipedia
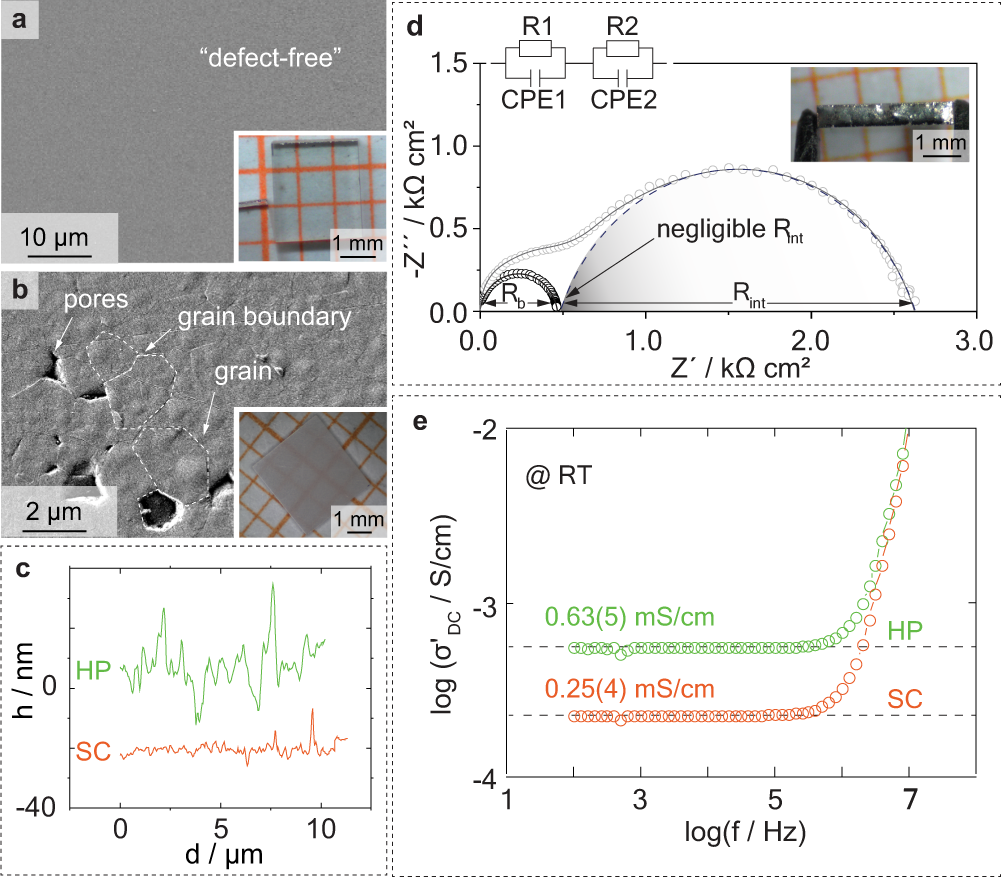
Effect of pulse-current-based protocols on the lithium dendrite

/energies/energies-15-05823/article_de

Compressibility of Liquids - an overview

Net-zero emissions chemical industry in a world of limited

Applied Sciences, Free Full-Text