Solved What is the equilibrium constant (Kp) at 45 °C for
Answer to Solved What is the equilibrium constant (Kp) at 45 °C for
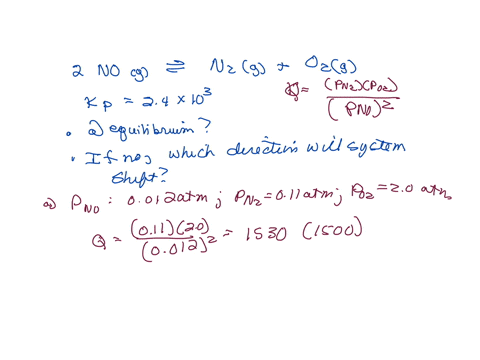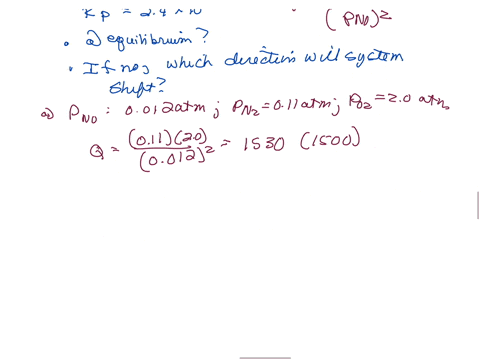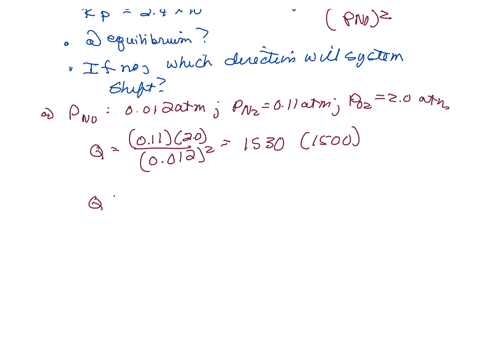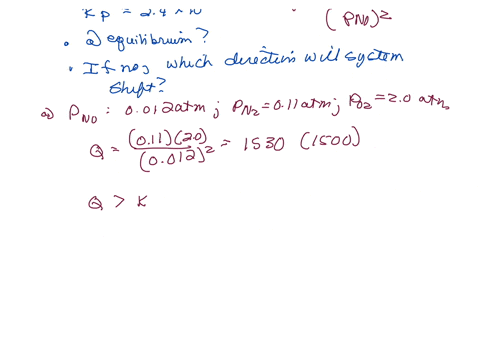
⏩SOLVED:The equilibrium constant Kp is 2.4 ×10^3 at a certain…

⏩SOLVED:As shown in Table 15.2, Kp for the equilibrium N2(g)+3 H2(g)…
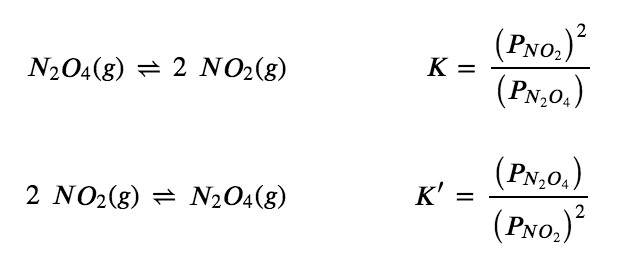
4.2 – The Equilibrium Constant & Reaction Quotient – General Chemistry for Gee-Gees
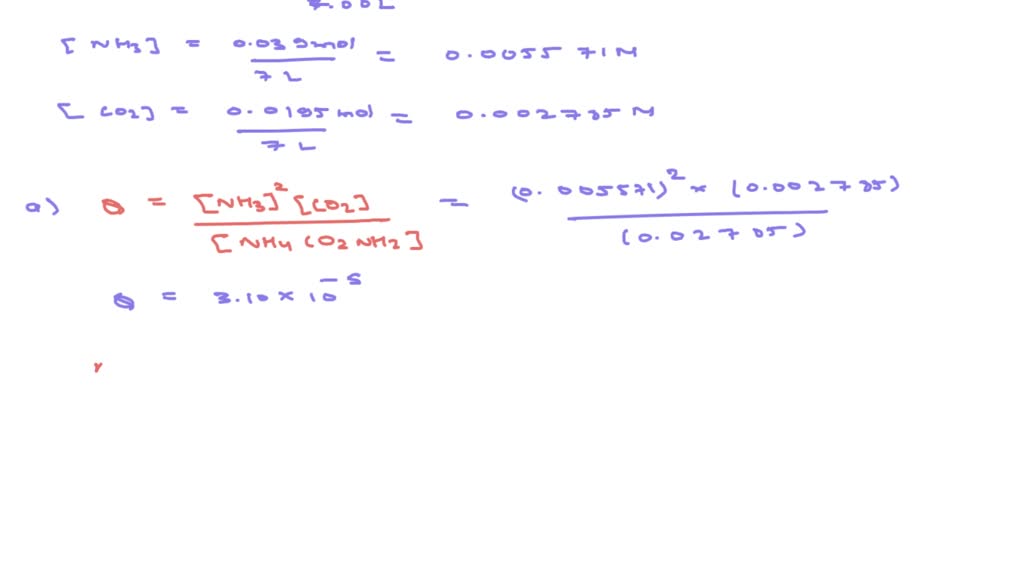
SOLVED: For the reaction below, the thermodynamic equilibrium constant is K = 1.33×10^(-2) at 45 °C. NH4CO2NH2(s) ⟶ 2NH3(g) + CO2(g) Suppose that 0.0085 moles of NH4CO2NH2, 0.017 moles of NH3, and

Calculating Equilibrium Constants (Part I)

The equilibrium constant (K_(p)) for the reaction, PCl_(5(g))hArrPCl_(3(g))+Cl_(2(g)) is 16. If

Calculating equilibrium constant Kp using partial pressures (article)

Solved 7. NOCI(g) = NO(g) + Cl2(g) Kp=1.7x10-2 at 45°C 8.

The equilibrium constant `K_(c)` for the following reaction at `842^(@)`C is `7.90xx10^(-3)`. What i

Solved Question 5 A reaction has an equilibrium constant of
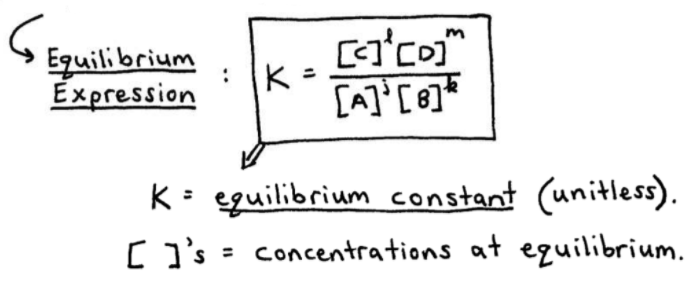
How to Calculate the Equilibrium Constant, K

The equilibrium constant (K(p)) at 27^(@)C for a homegenous gasesous r

16.6h Using the general properties of equilibrium constants
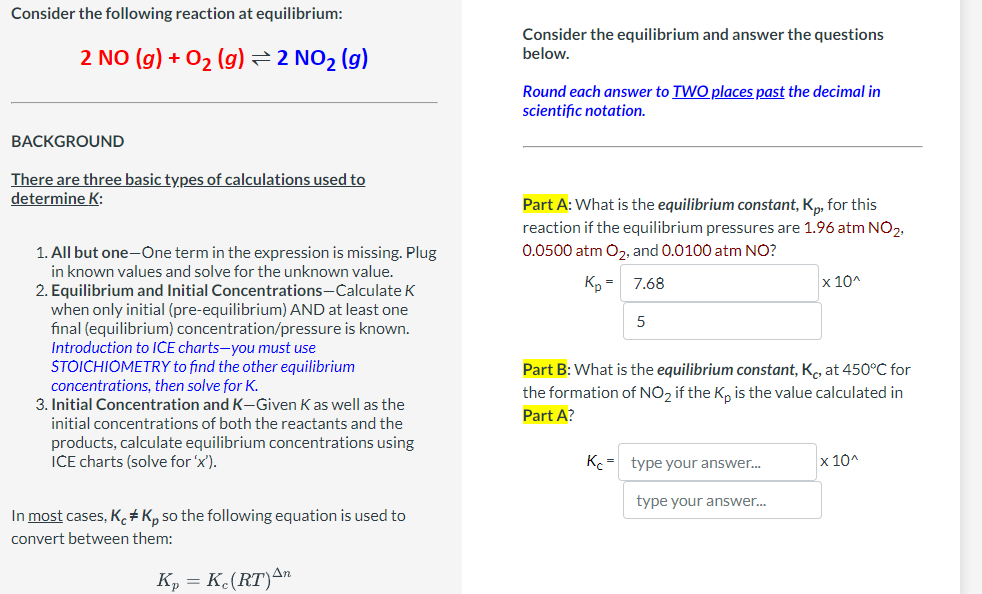
Solved What is the equilibrium constant, Kc, at 450°C for

The equilibrium constant (K_p) of the reaction N_2O_4 rightleftharpoons 2NO_2 was found to be 636mm 49.7^oC. Calculate the percentage dissociation of N_2O_4 when the pressure of the gas mixture is 182mm.