Isolation, expansion and characterization of porcine urinary bladder smooth muscle cells for tissue engineering, Biological Procedures Online
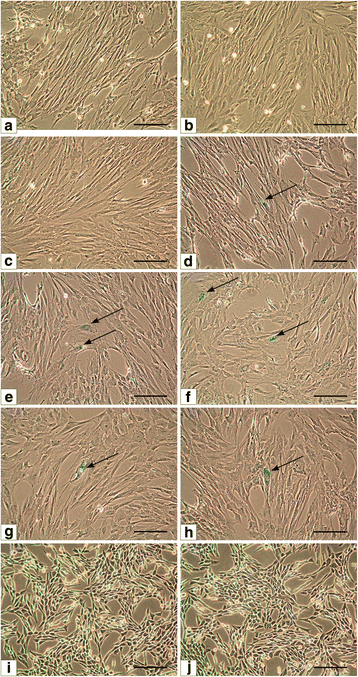
Background A key requirements for therapy utilizing the tissue engineering methodologies is use of techniques which have the capability to yield a high number of cells, from small tissue biopsy in a relatively short time. Up to date there was no optimal methods of isolation and expansion of urinary bladder smooth muscle cells (UB-SMCs). The aim of this study was to compare isolation and expansion techniques of UB-SMCs to select the most repeatable and efficient one. Method Five protocols of porcine UB- SMCs isolation including enzymatic and explant techniques and three expansion techniques were compared. Isolation effectiveness was evaluated using trypan blue assay. Cell phenotype was confirmed by immunofluorescence staining. Proliferation rate was analyzed using MTT and X- Celligence system. Cellular senescence was assessed measuring β-galactosidase activity. Results Enzymatic methods using collagenase with dispase (method I) or collagenase only (method III) allowed to isolate much larger number of cells than the methods using trypsin with collagenase (method II) and collagenase after digestion with trypsin (method IV). The success rate of establishment of primary culture was the highest when the isolated cells were cultured in the Smooth muscle Growth Medium-2 (SmGM-2). Expression of the smooth muscle markers- alpha smooth muscle actin and smoothelin was the highest for cells isolated by enzymatic method I and cultured in SmGM-2. There was no significant signs of cell senescence until the 8th passage. Conclusion The most efficient method of establishment of porcine UB-SMCs culture is enzymatic digestion of urinary bladder tissue with collagenase and dispase and culture of isolated cells in SmGM-2. This method was up to 10 times more efficient than other methods used for isolation and culture of UB-SMCs. This is an easy and consistent method for obtaining high numbers of urinary bladder smooth muscle cells.

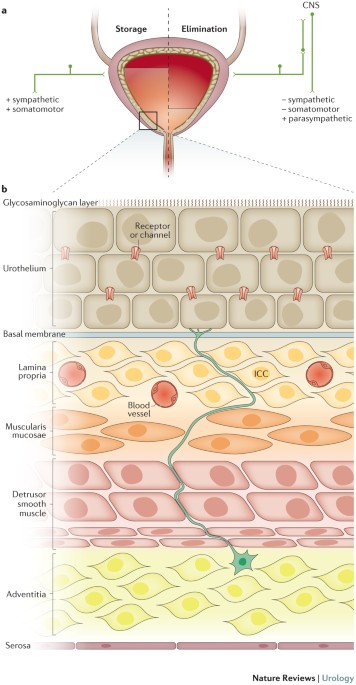
Location- and layer-dependent biomechanical and microstructural characterisation of the porcine urinary bladder wall - ScienceDirect

An examination of regenerative medicine-based strategies for the urinary bladder
IJMS, Free Full-Text

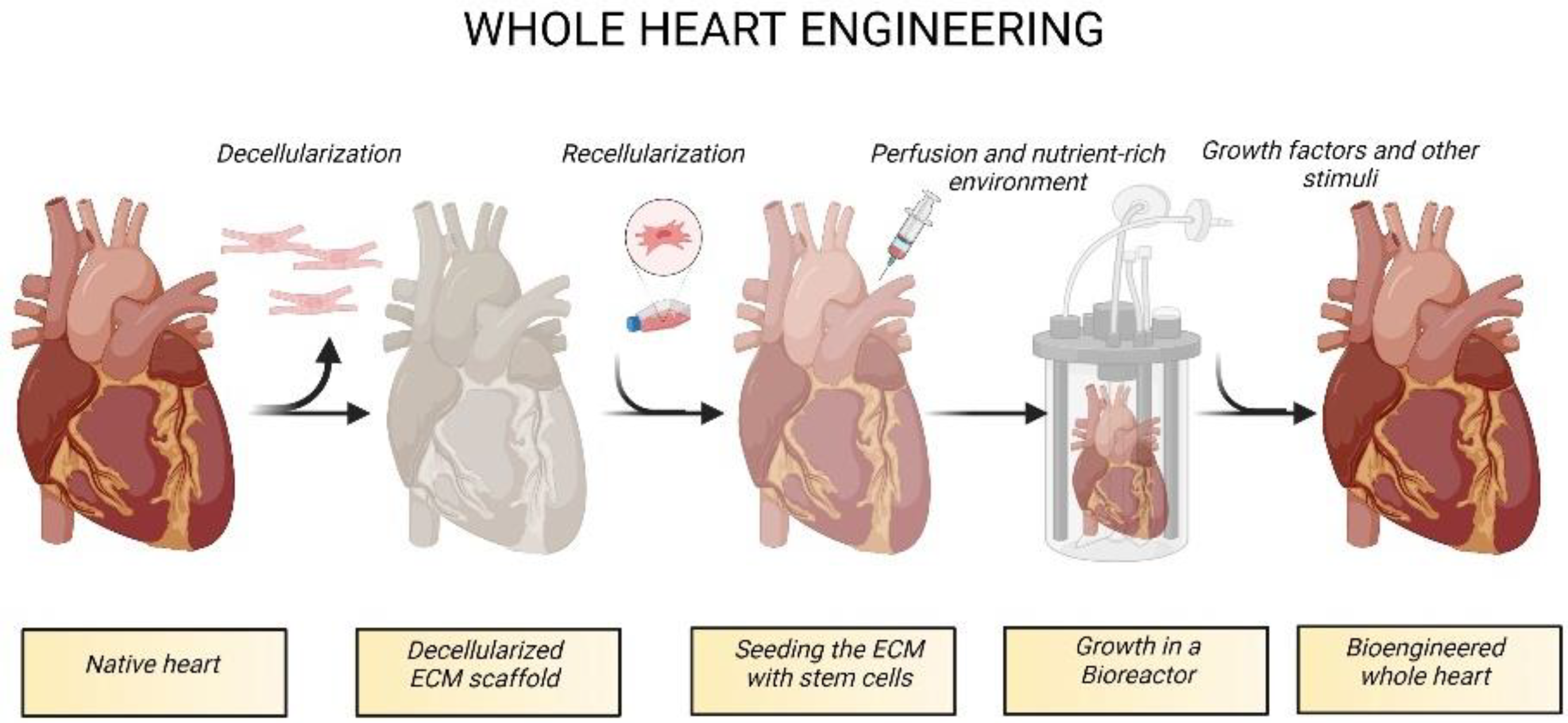
Decellularized extracellular matrix biomaterials for regenerative therapies: Advances, challenges and clinical prospects - ScienceDirect
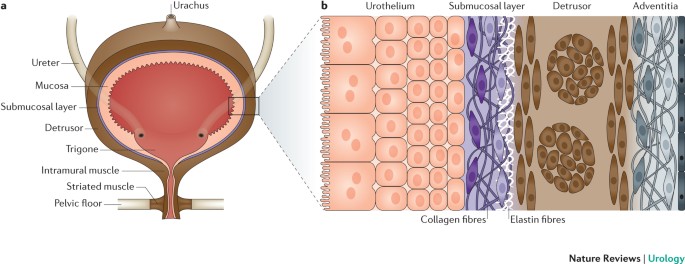
Bladder biomechanics and the use of scaffolds for regenerative medicine in the urinary bladder

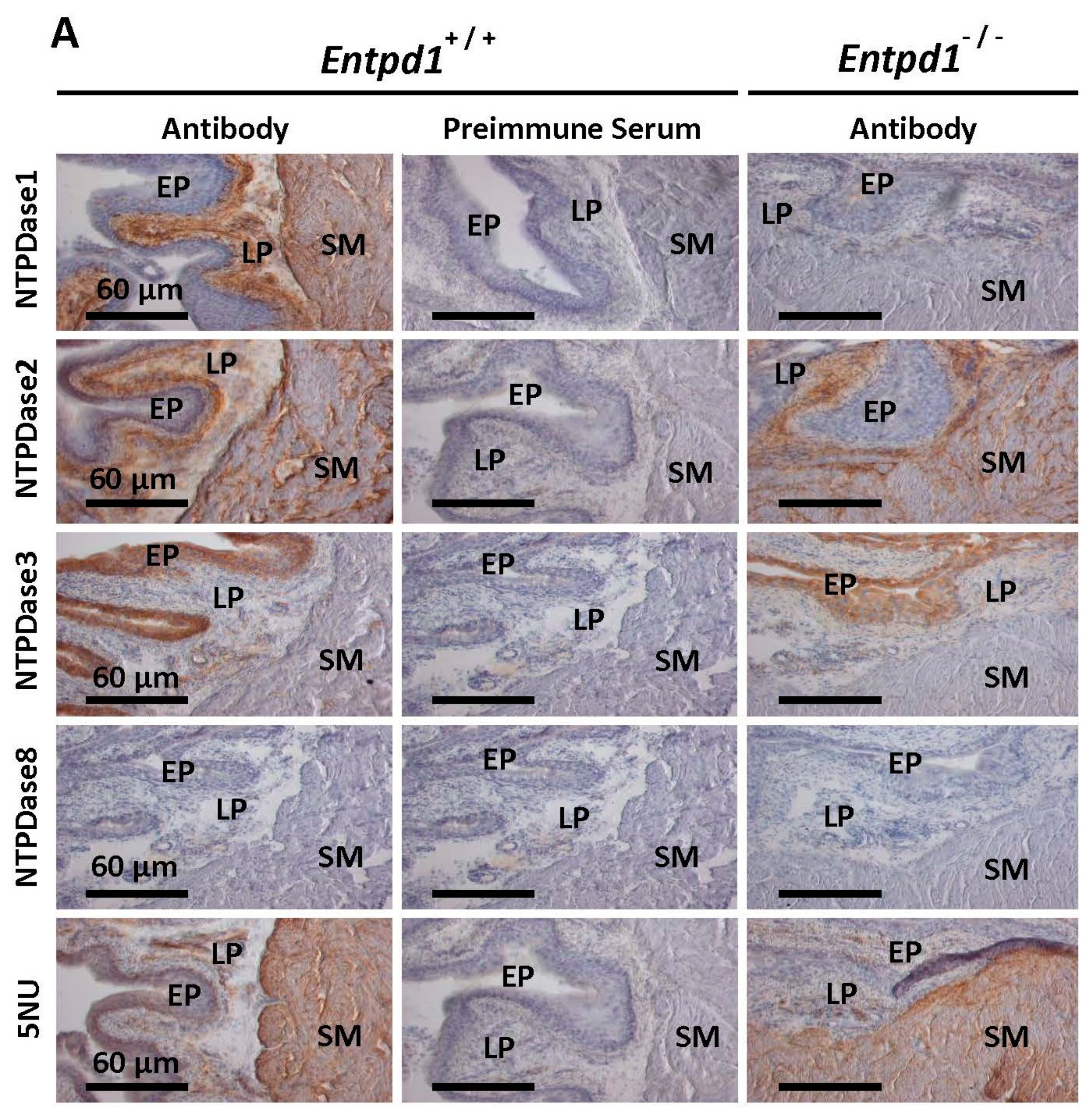
Mesenchymal stromal cells modulate the molecular pattern of healing process in tissue-engineered urinary bladder: the microarray data, Stem Cell Research & Therapy
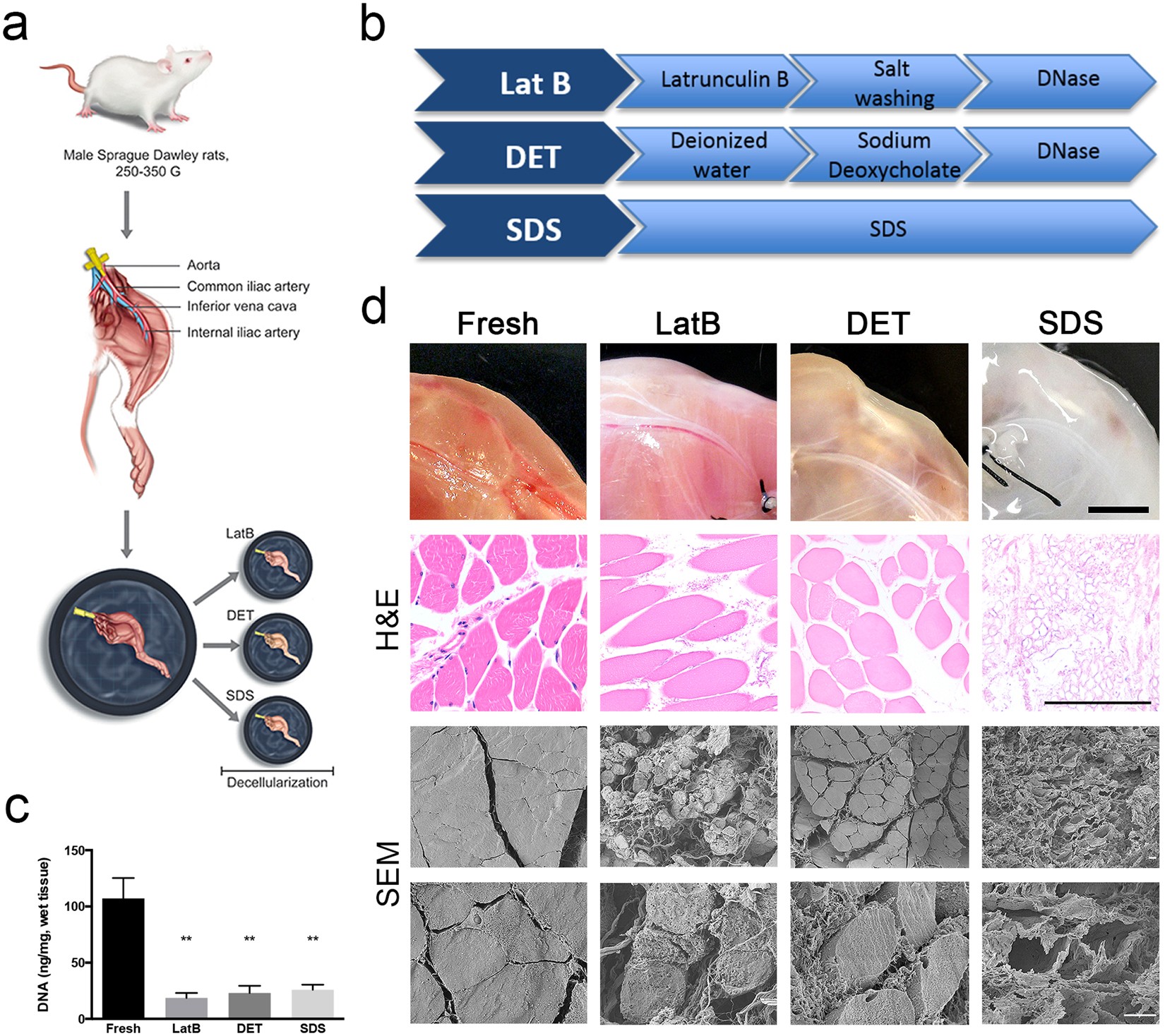
Decellularised skeletal muscles allow functional muscle regeneration by promoting host cell migration

Applied Sciences, Free Full-Text
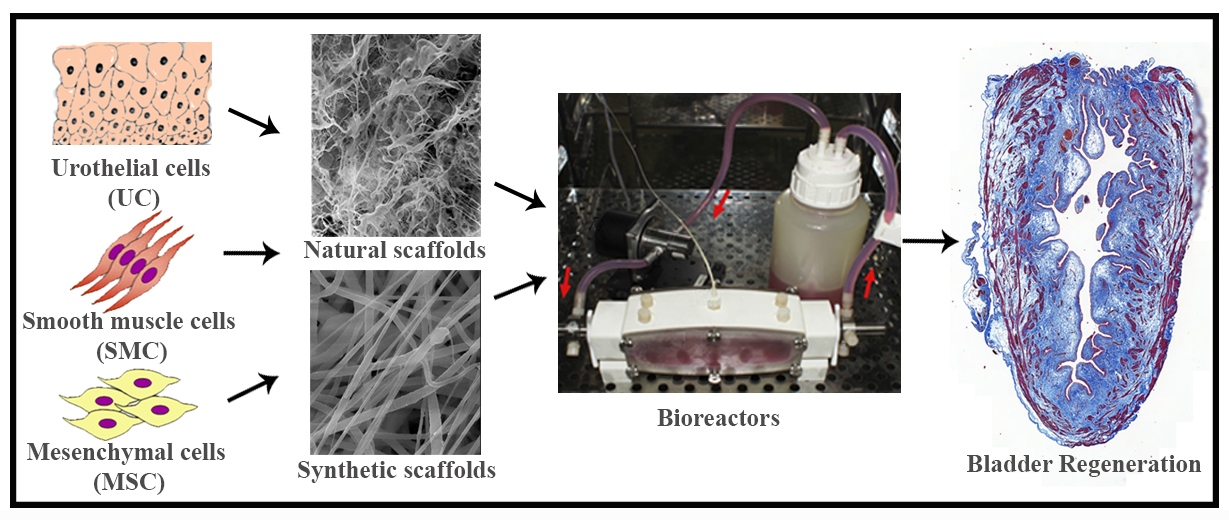
IJMS, Free Full-Text

Urinary bladder and urethral tissue engineering, and 3D bioprinting approaches for urological reconstruction
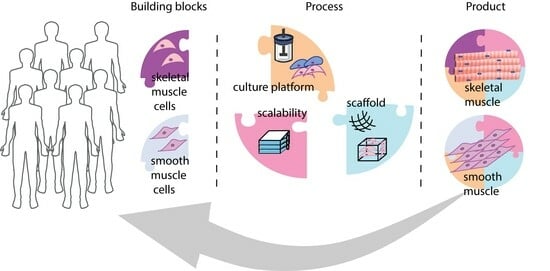
Bioengineering, Free Full-Text

Human embryonic stem cell-derived vascular smooth muscle cells in therapeutic neovascularisation - Journal of Molecular and Cellular Cardiology

IJMS, Free Full-Text
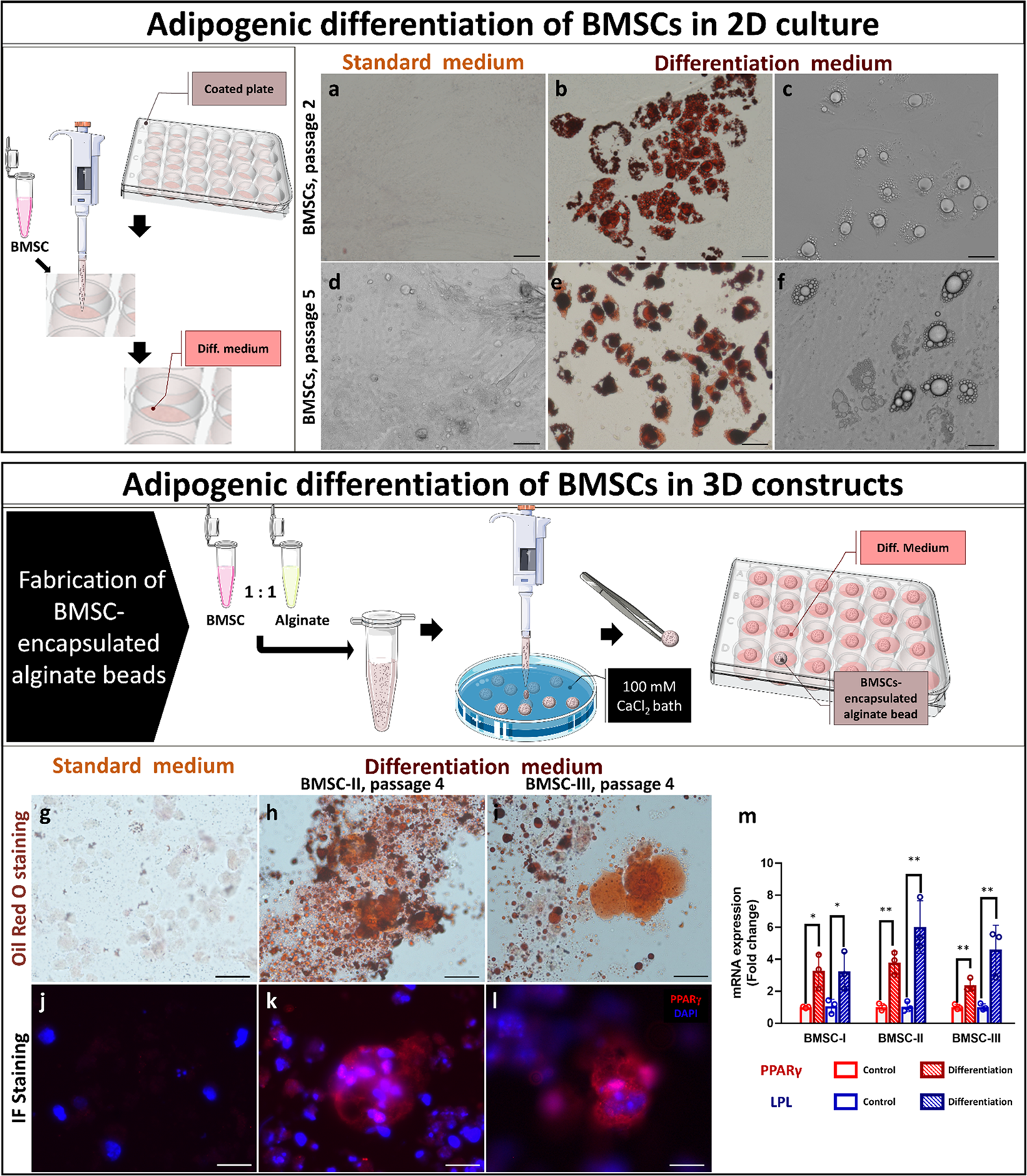
Engineered marble-like bovine fat tissue for cultured meat