physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange
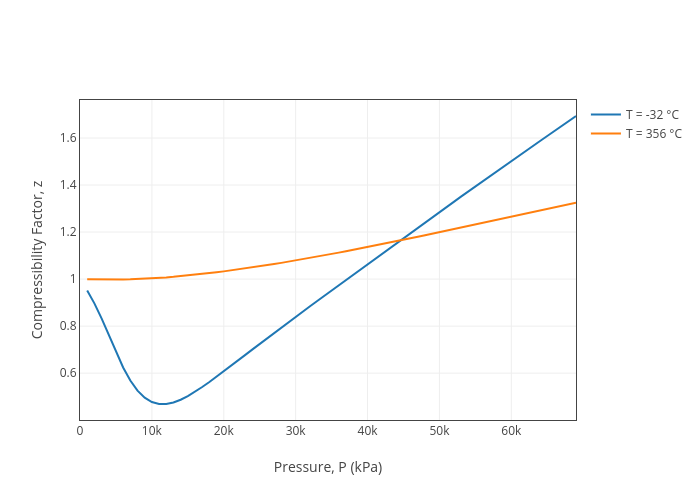
In the above graph,the minima of the curve for methane is more than that of nitrogen. Also, for a given value of pressure, the value of $Z$ for methane is less than that of nitrogen. They seem to m

Net-zero emissions chemical industry in a world of limited

Cold fusion - Wikipedia
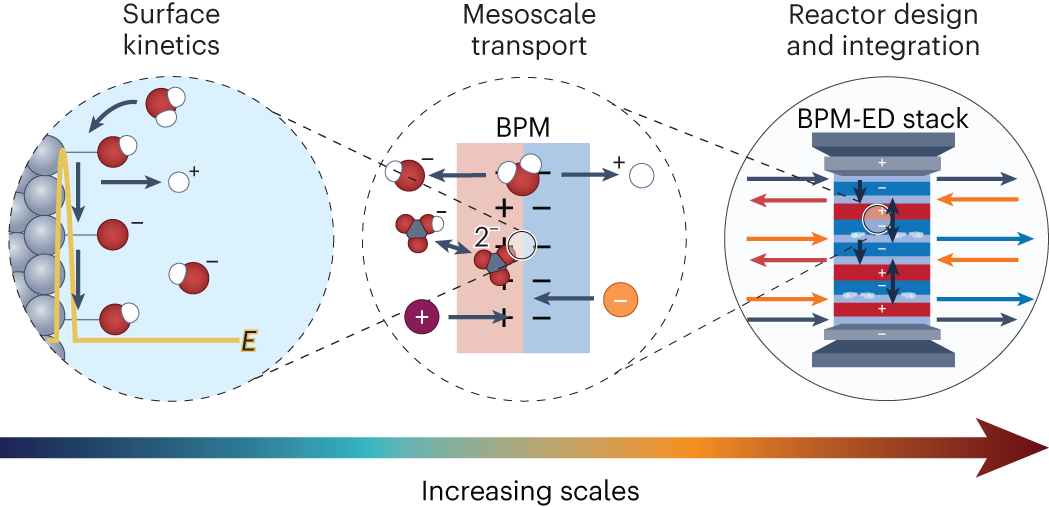
Multi-scale physics of bipolar membranes in electrochemical
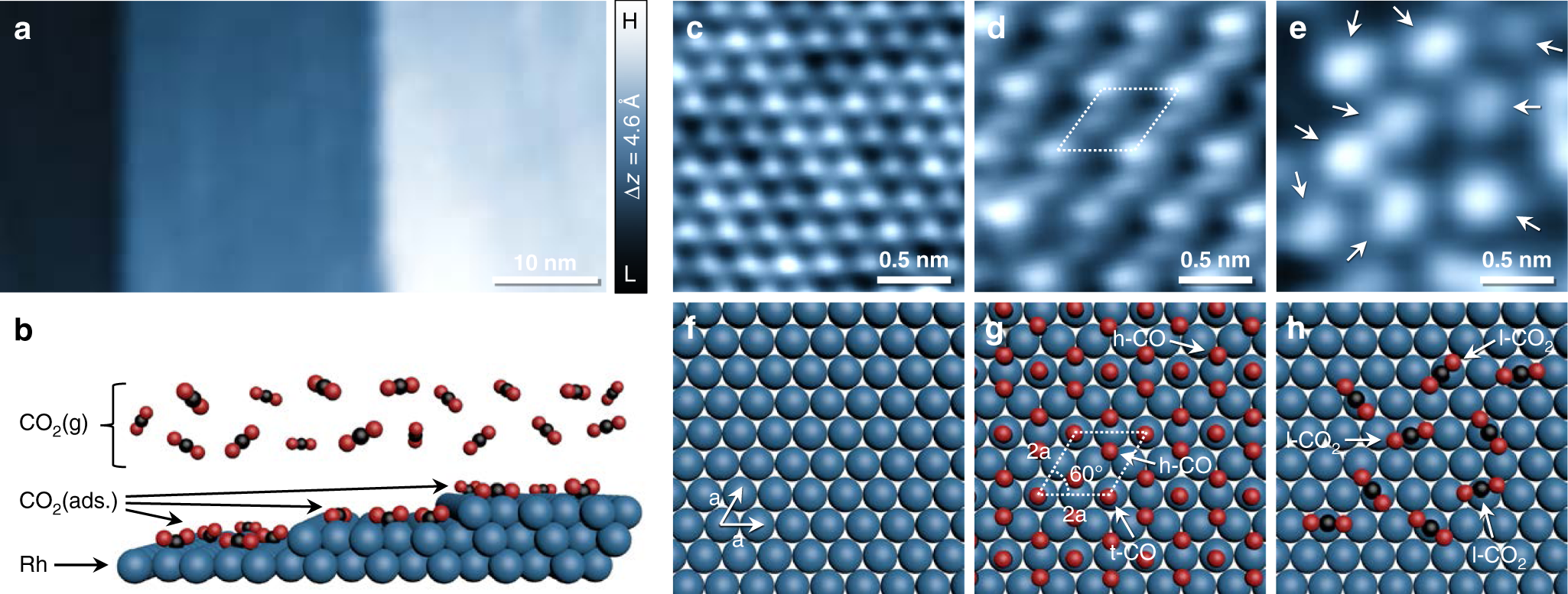
How Rh surface breaks CO2 molecules under ambient pressure

Minerals, Free Full-Text
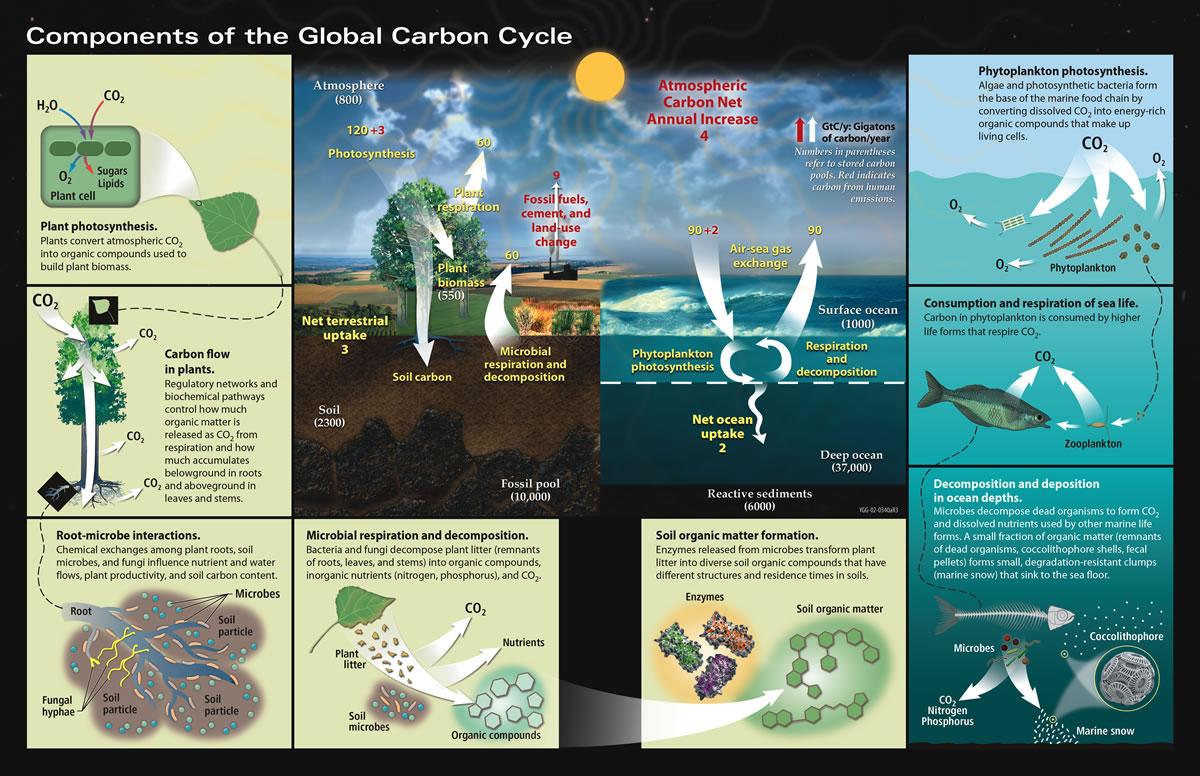
What is the Carbon Cycle? What is the science behind it?

Gas (Gaseous State) - Characteristics, Properties, Video, FAQs

Sustainability, Free Full-Text

Non-Ideal Gas Behavior Chemistry: Atoms First

Gas - Wikipedia
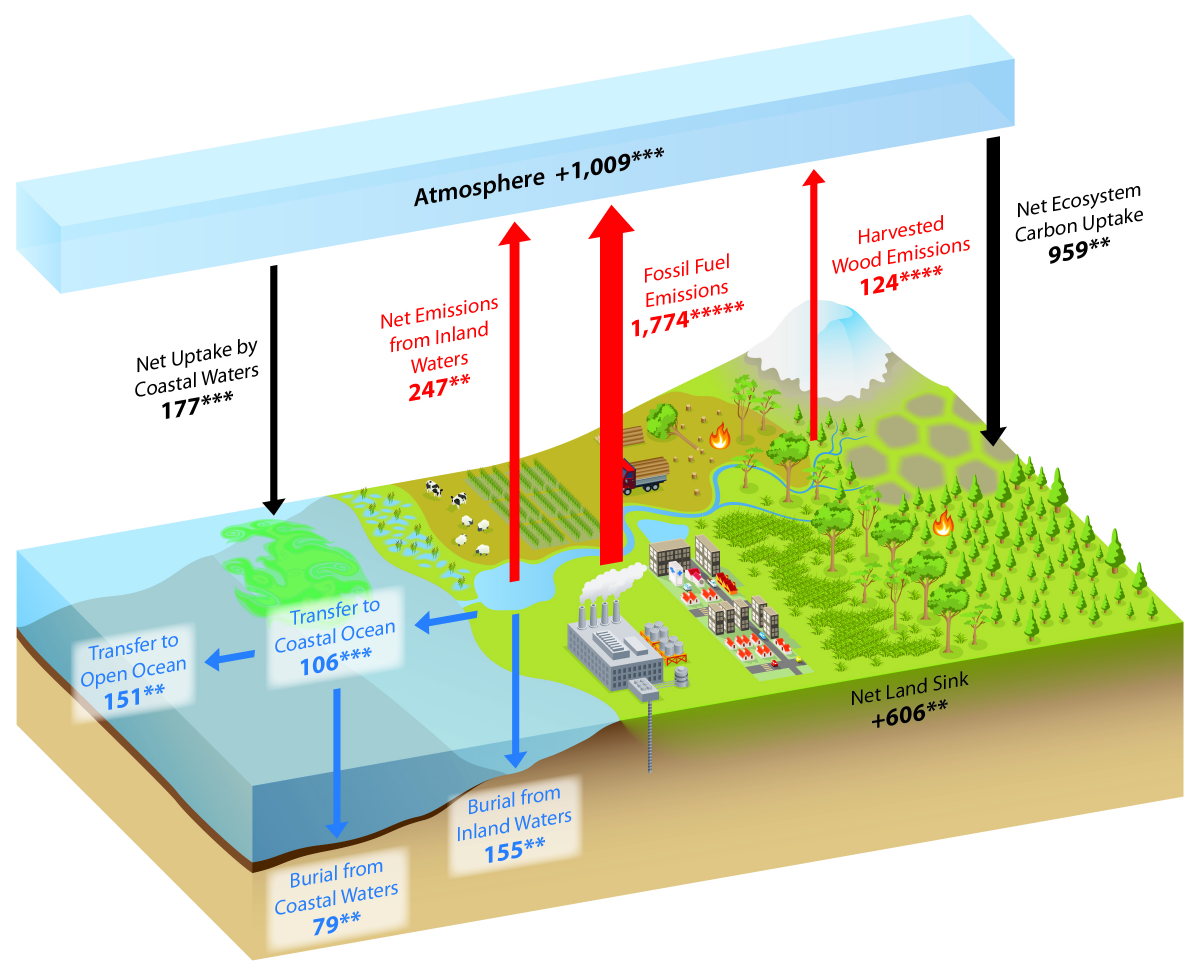
What is the Carbon Cycle? What is the science behind it?

A global equation-of-state model from mathematical interpolation

An overview on room-temperature chemiresistor gas sensors based on